Molar definition of molar explores the fundamental concepts of molar mass, molarity, moles, and molar volume, crucial for understanding chemical reactions and solutions. This comprehensive guide delves into calculating molar mass and molarity, explaining the significance of Avogadro’s number and how to use it in calculations. We’ll also explore the applications of these concepts in various scientific fields, including medicine and food science.
From understanding the relationship between moles and mass to calculating the molarity of solutions, this guide will break down these essential chemical concepts. We’ll provide clear examples, tables, and comparisons to ensure a thorough grasp of the material.
Molar Mass Definition
Molar mass is a fundamental concept in chemistry, crucial for understanding the amount of a substance and its composition. It quantifies the mass of one mole of a substance, providing a link between the microscopic world of atoms and molecules and the macroscopic world of laboratory experiments. This understanding is vital for stoichiometry calculations, which allow chemists to predict the amounts of reactants and products in chemical reactions.The molar mass of a substance is directly related to the number of moles present.
More specifically, one mole of a substance contains Avogadro’s number (approximately 6.022 x 10 23) of its constituent particles (atoms, molecules, or ions). This means that the molar mass, expressed in grams per mole (g/mol), represents the mass of Avogadro’s number of particles.
Definition of Molar Mass
Molar mass is the mass of one mole of a substance. It is numerically equivalent to the atomic weight (or formula weight) of the substance, but expressed in grams per mole (g/mol).
Relationship between Molar Mass and Moles
The molar mass provides a direct link between the mass of a substance and the number of moles. The number of moles (n) of a substance can be calculated by dividing the mass (m) of the substance by its molar mass (M). The formula is: n = m/M. This relationship is fundamental to quantitative analysis in chemistry, allowing us to convert between mass and the amount of substance in moles.
Units of Molar Mass
Molar mass is typically expressed in grams per mole (g/mol). This unit reflects the mass of one mole of a substance, measured in grams. The unit g/mol is essential for accurate calculations in chemical reactions and stoichiometry problems.
Calculating Molar Mass from Atomic Weights
To calculate the molar mass of a substance, you sum the atomic weights of all the atoms in the substance’s formula. The atomic weights are usually found on the periodic table and are expressed in atomic mass units (amu). For example, to calculate the molar mass of water (H 2O), we add the atomic weights of two hydrogen atoms and one oxygen atom: (2 x 1.008 amu) + (1 x 16.00 amu) = 18.02 g/mol.
The molar mass of water is 18.02 g/mol.
Molar Mass = Σ (Atomic Weight x Number of atoms)
Comparison of Molar Masses of Elements
The table below shows the molar masses of various elements, calculated using the atomic weights from the periodic table. These values are essential for various chemical calculations, including stoichiometry and reaction predictions.
| Element | Symbol | Atomic Weight (amu) | Molar Mass (g/mol) |
|---|---|---|---|
| Hydrogen | H | 1.008 | 1.008 |
| Carbon | C | 12.01 | 12.01 |
| Oxygen | O | 16.00 | 16.00 |
| Sodium | Na | 22.99 | 22.99 |
| Chlorine | Cl | 35.45 | 35.45 |
Molarity Definition
Molarity, a fundamental concept in chemistry, quantifies the concentration of a solute in a solution. It’s crucial for understanding how much of a substance is present in a given volume of liquid and is extensively used in various chemical calculations and experiments. A deep understanding of molarity is essential for navigating stoichiometry, reaction rates, and numerous other chemical phenomena.Molarity is a measure of the number of moles of solute per liter of solution.
This means that if you have a 1 molar (1M) solution of sodium chloride, for example, there is one mole of sodium chloride dissolved in every liter of the solution. This precise measurement is vital for ensuring reactions proceed as expected and for accurately determining the amounts of reactants and products involved.
Significance of Molarity in Chemistry
Molarity is a cornerstone of quantitative chemistry. It facilitates precise calculations in various chemical applications. It’s employed in titrations, where the concentration of an unknown solution is determined by reacting it with a solution of known molarity. It’s also critical in understanding reaction rates and equilibrium constants, providing insights into the behavior of chemical systems. Furthermore, molarity is vital for preparing solutions with specific concentrations, which is essential in many laboratory procedures.
Examples of Solutions with Varying Molarity Values
Different solutions exhibit varying molarity values depending on the amount of solute dissolved in a given volume of solvent. A 0.1 M solution of hydrochloric acid (HCl) contains 0.1 moles of HCl per liter of solution. Conversely, a 5 M solution of sulfuric acid (H₂SO₄) has 5 moles of H₂SO₄ per liter of solution. These differing molarity values directly influence the strength of the acid or base properties of the solution.
A highly concentrated solution (like the 5 M sulfuric acid) will have a significantly greater effect compared to a dilute solution (like the 0.1 M hydrochloric acid).
Comparison of Molarity with Other Concentration Units, Molar definition of molar
Molarity, expressed as moles per liter (mol/L), differs from other concentration units like molality (moles of solute per kilogram of solvent). While both describe solute concentration, molality is independent of temperature, making it suitable for certain applications where temperature variations might affect the volume of the solution. Molarity, on the other hand, is sensitive to temperature changes due to the volume dependency.
The choice of concentration unit depends on the specific needs of the application.
Table Illustrating Steps to Calculate Molarity
| Step | Description |
|---|---|
| 1 | Determine the moles of solute. This involves using the formula: moles = mass / molar mass. |
| 2 | Calculate the volume of the solution in liters. Ensure the volume is in liters, not milliliters. |
| 3 | Divide the moles of solute by the volume of the solution in liters. The result is the molarity of the solution. |
| 4 | Express the molarity using the unit mol/L or M. |
Molarity (M) = moles of solute / liters of solution
Moles and Avogadro’s Number
The mole is a fundamental concept in chemistry, providing a way to count incredibly large numbers of atoms or molecules. It’s crucial for understanding the quantitative relationships in chemical reactions and calculations. This section delves into the definition of a mole, Avogadro’s number, and the connections between moles, mass, and the number of particles.Understanding the relationship between the number of particles, mass, and the amount of substance is essential for various chemical calculations.
This understanding allows us to accurately predict reaction outcomes and analyze the composition of matter.
The Mole Concept
The mole is a unit of measurement representing the amount of substance. One mole of any substance contains a fixed number of particles, a concept directly tied to Avogadro’s number. This fixed number of particles is crucial for relating macroscopic observations (like mass) to microscopic entities (atoms and molecules).
Avogadro’s Number
Avogadro’s number, approximately 6.022 x 10 23, is the number of particles (atoms, molecules, ions, or other entities) in one mole of a substance. Its significance lies in its ability to connect the microscopic world of atoms and molecules to the macroscopic world of measurable quantities like mass. This constant provides a bridge between the atomic scale and the scale we can work with in the laboratory.
Relationship Between Moles, Mass, and Number of Particles
The mole concept establishes a direct relationship between the amount of substance (in moles), its mass (in grams), and the number of particles (atoms or molecules). This relationship is essential for performing stoichiometric calculations and understanding the composition of chemical compounds.
Number of moles = Mass (grams) / Molar Mass (grams/mole)
Number of particles = Number of moles
Avogadro’s number
Calculating the Number of Particles
To calculate the number of particles in a given mass of a substance, you first determine the number of moles using the substance’s molar mass. Then, multiply the number of moles by Avogadro’s number.
-
Example 1: Calculate the number of atoms in 10 grams of sodium (Na). Sodium’s molar mass is approximately 23 g/mol.
First, calculate the number of moles: 10 g / 23 g/mol ≈ 0.435 moles.
Then, calculate the number of atoms: 0.435 moles
– 6.022 x 10 23 atoms/mole ≈ 2.62 x 10 23 atoms. -
Example 2: How many molecules are present in 34.0 grams of ammonia (NH 3)?
Ammonia’s molar mass is approximately 17.03 g/mol.
First, calculate the number of moles: 34.0 g / 17.03 g/mol ≈ 1.999 moles.
Then, calculate the number of molecules: 1.999 moles
– 6.022 x 10 23 molecules/mole ≈ 1.20 x 10 24 molecules.
Connection Between Moles, Mass, and Number of Particles (Table)
The following table illustrates the interrelationship between moles, mass, and the number of particles for various substances. It highlights the crucial role of molar mass in converting between these quantities.
| Substance | Molar Mass (g/mol) | Mass (grams) | Number of Moles | Number of Particles |
|---|---|---|---|---|
| Water (H2O) | 18.015 | 36.03 | 2 | 1.204 x 1024 |
| Carbon Dioxide (CO2) | 44.01 | 88.02 | 2 | 1.204 x 1024 |
| Glucose (C6H12O6) | 180.16 | 360.32 | 2 | 1.204 x 1024 |
Molar Volume of Gases
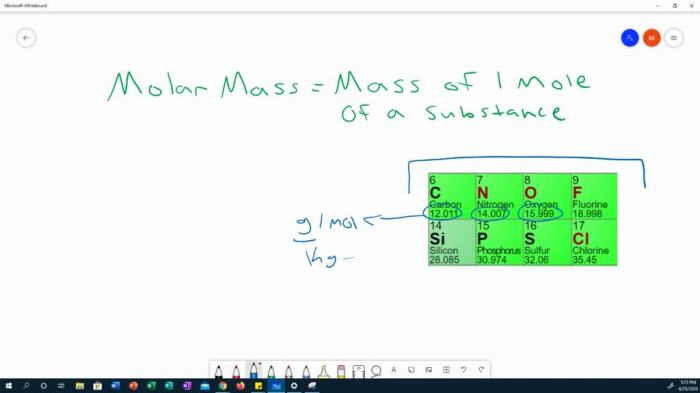
The molar volume of a gas is a crucial concept in chemistry, allowing us to relate the amount of a gas (in moles) to its volume. Understanding this relationship is essential for various applications, from stoichiometry calculations to gas law experiments. This concept is closely tied to the ideal gas law and plays a vital role in predicting gas behavior under specific conditions.
Definition of Molar Volume
The molar volume of a gas is the volume occupied by one mole of a gas at a given temperature and pressure. Crucially, this volume is a characteristic property that depends on the external conditions (temperature and pressure). This characteristic is a significant parameter for understanding and predicting gas behavior.
Relationship to the Ideal Gas Law
The molar volume is intrinsically linked to the ideal gas law, which describes the relationship between pressure (P), volume (V), number of moles (n), and temperature (T) of an ideal gas. The ideal gas law is expressed as PV = nRT, where R is the ideal gas constant. Rearranging this equation to solve for volume per mole (V/n) gives us the molar volume (V m).
The equation becomes V m = RT/P. This direct relationship highlights how the molar volume is directly proportional to temperature and inversely proportional to pressure. The formula clearly demonstrates the influence of external conditions on the molar volume.
Conditions for Constant Molar Volume
The molar volume of a gas is constant only under specific conditions. These conditions are when the gas behaves ideally. This means that the gas molecules occupy negligible volume compared to the total volume, and there are no intermolecular forces acting between the molecules. In reality, these ideal conditions are seldom perfectly met, but gases often behave nearly ideally under certain circumstances.
For example, at low pressures and high temperatures, the deviation from ideal behavior is minimal.
Calculating Gas Volume
To calculate the volume of a gas given its moles, you need to know the temperature, pressure, and the number of moles of the gas. Use the ideal gas law, plugging in the known values, and solve for the volume (V). For instance, if you have 2 moles of a gas at 273 K and 1 atm pressure, using the ideal gas law, we can calculate the volume.
Examples of Calculation
Let’s consider an example. Calculate the volume occupied by 0.5 moles of nitrogen gas at standard temperature and pressure (STP). STP conditions are defined as 0°C (273 K) and 1 atm pressure. Using the ideal gas law (PV = nRT) and substituting the known values, we can calculate the volume. With R = 0.0821 L·atm/mol·K, the volume is calculated as V = (0.5 mol
- 0.0821 L·atm/mol·K
- 273 K) / 1 atm = 11.2 liters.
Summary Table
| Conditions | Molar Volume (L/mol) | Notes |
|---|---|---|
| Standard Temperature and Pressure (STP) | 22.4 | 0°C (273 K) and 1 atm |
| 25°C (298 K) and 1 atm | 24.5 | Common room temperature and pressure |
| 0°C (273 K) and 2 atm | 11.2 | Higher pressure than STP |
Molar Concentration in Different Situations
Molar concentration, or molarity, is a fundamental concept in chemistry, quantifying the amount of solute dissolved in a given volume of solution. Understanding molarity in various scenarios is crucial for a wide range of applications, from preparing solutions for experiments to analyzing chemical reactions. This exploration delves into how molarity behaves in different solution types, and its essential role in chemical calculations.Molarity, expressed as moles of solute per liter of solution (mol/L), provides a standardized way to compare the concentration of different solutions.
Understanding the molar definition is key, but did you know that similar complexities exist in the world of human health? For example, knowing the different types of lung cancer, like small cell and non-small cell lung cancer, types of lung cancer , can be just as important as grasping the molar definition of molar. Ultimately, understanding the intricacies of both scientific and health-related concepts is essential for a well-rounded perspective.
This is why exploring molar definition is important in its own right.
It’s vital to recognize that the volume used in calculating molarity is the total volume of the solution, not just the volume of the solvent. This distinction is critical when dealing with solutions where the volume of the solute significantly impacts the final volume.
Molarity in Different Types of Solutions
Different types of solutions exhibit varying characteristics in terms of molarity. For example, concentrated solutions contain a high amount of solute relative to the solvent, while dilute solutions have a low amount of solute. The concentration of a solution can significantly affect its physical properties, such as its boiling point and freezing point.
Expressing Molarity in Different Contexts
Molarity is frequently expressed in different units depending on the context. For instance, in laboratory settings, molarity is often represented using the capital letter “M” (e.g., 2.5 M HCl). In some industrial applications, or scientific publications, molarity may be expressed in millimoles per liter (mmol/L) or micromoles per liter (µmol/L) to represent very dilute solutions. These variations in units reflect the need for precision and convenience in specific applications.
Comparing Molarity in Solutions with Different Solvents
The choice of solvent can influence the molarity of a solution. Different solvents have varying densities, which in turn affects the volume of the solution. Therefore, while the amount of solute (in moles) remains constant, the molarity can differ between solutions of the same solute dissolved in different solvents. A solution prepared in a denser solvent will result in a lower molarity for the same amount of solute compared to a solution in a less dense solvent.
Molar Concentration in Chemical Reactions
Molarity plays a critical role in understanding and performing calculations related to chemical reactions. The balanced chemical equation for a reaction provides the stoichiometric ratios of reactants and products. Knowing the molarity of the reactants allows us to calculate the moles of each reactant present. Using these moles and the stoichiometric ratios, we can predict the moles of products formed or the moles of reactants consumed.
Understanding the molar definition of molar mass is crucial for various applications, but did you know it also indirectly connects to the complexities of mental health? For instance, precise dosage calculations in medications for anxiety and depression like these rely heavily on molar concentrations. Ultimately, grasping molar mass concepts is key to understanding how many molecules are present, which in turn influences treatment strategies in many ways.
Role of Molar Concentration in Stoichiometric Calculations
Stoichiometric calculations heavily rely on molarity. By knowing the molarity of the reactants and the balanced equation, we can calculate the theoretical yield of a reaction. The limiting reactant, the reactant that gets completely consumed first, determines the maximum amount of product that can be formed. This principle is crucial in designing efficient chemical processes, where maximizing product yield is paramount.
Example: If a reaction requires 2 moles of reactant A for every 1 mole of reactant B, and the molarity of A is 2.5 M and the molarity of B is 1.0 M, we can determine the limiting reactant and predict the maximum yield of the product.
Molar Mass Calculations
Unveiling the weight of a mole! Understanding molar mass is crucial for countless chemical calculations. It bridges the gap between the microscopic world of atoms and molecules and the macroscopic world of laboratory experiments. This section delves into the nitty-gritty of calculating molar masses, demonstrating how to utilize periodic tables and chemical formulas to determine the mass of a mole of any substance.Calculating molar mass is a fundamental skill in chemistry.
It allows chemists to relate the number of particles in a substance to its mass, essential for stoichiometry, solution preparation, and countless other applications. Knowing the molar mass of a compound is akin to having a conversion factor between the number of molecules and the weight of a sample.
Calculating Molar Mass from Chemical Formulas
To calculate the molar mass of a compound, we need to know its chemical formula. This formula reveals the types and numbers of atoms present in one molecule of the substance. The molar mass is the sum of the atomic masses of all atoms in the formula.
Molar Mass = Σ (Atomic Mass × Number of atoms)
For example, consider water (H 2O). Its chemical formula tells us that one molecule of water contains two hydrogen atoms and one oxygen atom. Using the periodic table, the atomic mass of hydrogen (H) is approximately 1.01 amu, and the atomic mass of oxygen (O) is approximately 16.00 amu. Applying the formula:Molar Mass (H 2O) = (2 × 1.01 amu) + (1 × 16.00 amu) = 18.02 amuTherefore, the molar mass of water is 18.02 g/mol.
Using Periodic Tables in Molar Mass Calculations
Periodic tables are indispensable tools in chemistry. They list the atomic masses of all known elements. To find the atomic mass of an element, locate the element on the periodic table and find the value reported below its symbol. This value represents the average atomic mass of the naturally occurring isotopes of that element, expressed in atomic mass units (amu) or grams per mole (g/mol).
Examples of Molar Mass Calculations
Let’s illustrate with more examples:
- Carbon Dioxide (CO2): Carbon (C) has an atomic mass of approximately 12.01 amu, and oxygen (O) has an atomic mass of approximately 16.00 amu. Thus, the molar mass of CO 2 is (1 × 12.01 amu) + (2 × 16.00 amu) = 44.01 g/mol.
- Sodium Chloride (NaCl): Sodium (Na) has an atomic mass of approximately 22.99 amu, and chlorine (Cl) has an atomic mass of approximately 35.45 amu. Therefore, the molar mass of NaCl is (1 × 22.99 amu) + (1 × 35.45 amu) = 58.44 g/mol.
- Glucose (C6H 12O 6): Calculating the molar mass of glucose involves summing the atomic masses of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. The result is (6 × 12.01 amu) + (12 × 1.01 amu) + (6 × 16.00 amu) = 180.18 g/mol.
Molar Mass Calculation Table
The table below summarizes the molar mass calculations for various chemical compounds.
| Compound | Formula | Molar Mass (g/mol) |
|---|---|---|
| Water | H2O | 18.02 |
| Carbon Dioxide | CO2 | 44.01 |
| Sodium Chloride | NaCl | 58.44 |
| Glucose | C6H12O6 | 180.18 |
| Ethanol | C2H5OH | 46.07 |
Significance of Significant Figures in Molar Mass Calculations
Significant figures are crucial in chemical calculations, including molar mass calculations. The number of significant figures in the molar mass should match the least precise atomic mass used in the calculation. This ensures that the final result reflects the accuracy of the measurements or data used.
So, a molar, in chemistry, is a unit of measurement for a substance’s amount. But, if you’re wondering about the nutritional benefits of bone broth, a topic I’ve explored a lot lately, check out this article on is bone broth good for you. While bone broth isn’t a direct application of molar definitions, it’s interesting to consider the amounts of minerals and nutrients it might contain in relation to molarity, as different nutrients have different molar masses.
Ultimately, though, a molar definition is about the quantity of a substance, and that’s a key concept in chemistry.
Molarity Calculations and Examples
Molarity, a crucial concept in chemistry, quantifies the concentration of a solute in a solution. It’s defined as the number of moles of solute per liter of solution. Understanding molarity calculations is essential for various applications, from preparing specific solutions in a lab to analyzing the concentration of substances in environmental samples or biological fluids. Accurate calculations rely on precise understanding of the units involved.Calculating molarity involves a straightforward formula, but meticulous attention to units is critical.
This section delves into practical examples, demonstrating how to calculate molarity for different solutions, determining the necessary solute mass for specific molar solutions, and highlights the importance of unit consistency. It also Artikels the steps for preparing solutions with desired molarity and different ways to express solution concentration using molarity.
Calculating Molarity
Molarity (M) is calculated by dividing the moles of solute by the volume of the solution in liters. The formula is:
M = moles of solute / liters of solution
Example 1: Calculating Molarity
Suppose you dissolve 0.5 moles of sodium chloride (NaCl) in 250 milliliters of water. First, convert the volume to liters: 250 mL = 0.250 L. Applying the formula: M = 0.5 moles / 0.250 L = 2.0 M. Thus, the molarity of the solution is 2.0 M.
Example 2: Determining Solute Mass
To prepare a 0.50 M solution of sulfuric acid (H 2SO 4), you need 250 mL of solution. First, calculate the moles of solute required: Moles = Molarity × Volume (in liters) = 0.50 M × 0.250 L = 0.125 moles. Next, find the molar mass of H 2SO 4 (approximately 98.08 g/mol). Then, calculate the mass of H 2SO 4 needed: Mass = Moles × Molar Mass = 0.125 moles × 98.08 g/mol = 12.26 g.
Therefore, 12.26 grams of sulfuric acid are needed to prepare the solution.
Importance of Units in Molarity Calculations
Maintaining consistent units is paramount in molarity calculations. Converting volumes from milliliters to liters and ensuring that the solute’s amount is in moles is crucial for obtaining the correct molarity value. Incorrect unit conversions lead to significant errors in the calculation.
Steps for Preparing a Solution with Specific Molarity
- Calculate the required moles of solute using the desired molarity and solution volume.
- Determine the mass of solute needed by multiplying the moles of solute by the molar mass of the solute.
- Accurately measure the calculated mass of solute using a balance.
- Transfer the solute to a volumetric flask.
- Add a small amount of solvent to dissolve the solute completely.
- Add more solvent until the solution reaches the mark on the volumetric flask.
- Mix thoroughly to ensure uniform distribution of the solute.
Different Ways to Express Solution Concentration Using Molarity
Molarity is a convenient way to express the concentration of a solution. Other ways include mass percent, parts per million (ppm), and parts per billion (ppb). Each method provides a different perspective on the composition of the solution, with molarity focusing on the number of moles per liter. For example, a 1 M solution of sodium chloride has a different mass percent than a 1 M solution of glucose, highlighting the distinct nature of each concentration unit.
Applications of Molar Concepts: Molar Definition Of Molar
Molar concepts, encompassing molar mass, molarity, and moles, are fundamental tools in chemistry, enabling us to quantify and understand the behavior of matter at a molecular level. These concepts provide a bridge between the macroscopic world of measurable quantities and the microscopic world of atoms and molecules. This section explores the practical applications of molar concepts in diverse fields, demonstrating their significance in various scientific and technological endeavors.
Molar Mass in Various Fields
Molar mass, the mass of one mole of a substance, is a critical parameter in numerous fields. In medicine, it’s essential for calculating dosages of medications accurately. For example, determining the molar mass of a drug allows pharmacists to precisely measure the required amount for a patient, preventing overdose or underdosage. In food science, molar mass is crucial for understanding nutrient content and for formulating recipes.
Nutritional information often uses molar quantities to describe the amount of vitamins and minerals present in a food item. In material science, molar mass helps in designing and synthesizing new materials with specific properties. Understanding the molar mass of components allows for precise control over the composition of composite materials, leading to better performance.
Molarity in Titrations and Analytical Techniques
Molarity, a measure of concentration, plays a pivotal role in analytical chemistry. Titrations, a common analytical technique, rely heavily on molarity. By precisely measuring the volume of a solution of known molarity required to react completely with a solution of unknown concentration, the unknown concentration can be calculated. This technique is widely used in various applications, including determining the acidity of a sample or the concentration of a specific substance in a mixture.
Other analytical techniques, like spectrophotometry, also utilize molarity to relate the measured absorbance or transmission of light to the concentration of the analyte.
Molar Concepts in Chemical Reactions and Stoichiometry
Chemical reactions involve the rearrangement of atoms, and molar concepts are crucial for understanding the quantitative relationships between reactants and products. Stoichiometry, the calculation of reactants and products in chemical reactions, relies heavily on the concept of moles. The balanced chemical equation provides the mole ratios, allowing chemists to predict the amounts of reactants needed or the amounts of products formed.
For instance, knowing the molar ratio of reactants in a combustion reaction allows engineers to design more efficient engines.
Significance of Molar Calculations in Understanding Chemical Processes
Molar calculations are essential for understanding and predicting chemical processes. By relating macroscopic quantities to microscopic entities, molar calculations provide a quantitative framework for studying reactions, equilibrium, and kinetics. Calculations involving molar mass, molarity, and mole ratios provide insights into the quantitative relationships between reactants and products, enabling predictions about reaction yields and reaction pathways. For example, understanding the molar ratios of reactants and products in a chemical reaction allows for optimization of manufacturing processes.
Summary Table of Applications
| Field | Application of Molar Concepts |
|---|---|
| Medicine | Calculating drug dosages, understanding drug interactions |
| Food Science | Determining nutrient content, formulating recipes |
| Material Science | Designing new materials with specific properties, controlling material composition |
| Analytical Chemistry | Performing titrations, determining concentrations using spectrophotometry |
| Chemical Engineering | Optimizing chemical processes, predicting reaction yields |
Last Word
In conclusion, mastering molar concepts is essential for navigating the world of chemistry. Understanding molar mass, molarity, moles, and molar volume empowers you to analyze chemical reactions, calculate concentrations, and predict outcomes. This guide serves as a robust foundation for further exploration in chemistry and related disciplines. We hope this overview has been helpful in your journey to understanding these critical concepts.